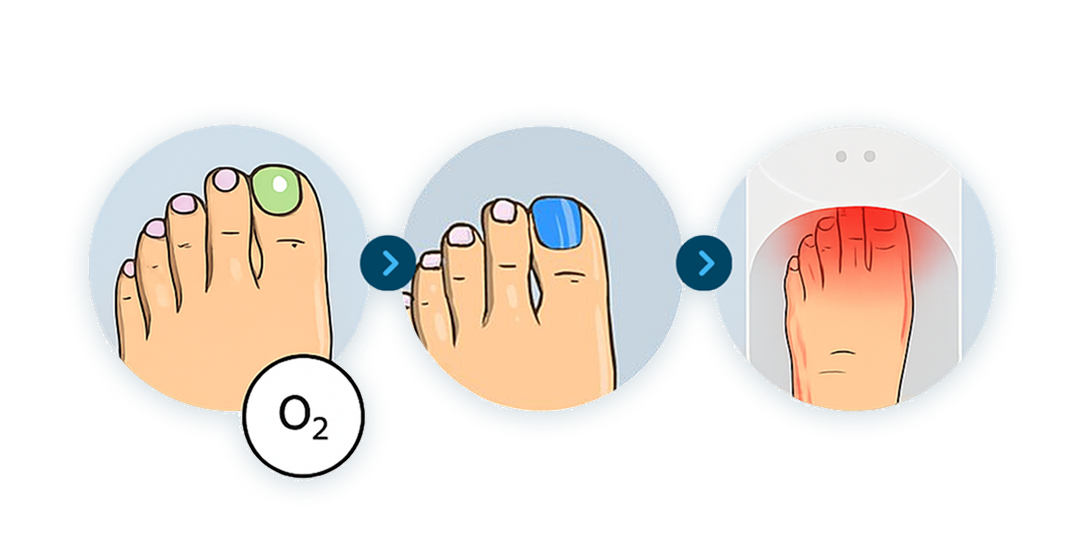
The Power of ToeFX Therapy Light
The light source transfers energy to the affected area of the nail, disrupting fungal structures at the cellular level. When used with the ToeFX topical formulation, which helps visualize the treatment area, the system delivers a precise, light-activated approach to managing nail health - all without drugs, discomfort, or downtime.
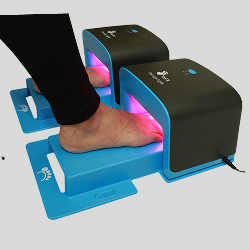
The ToeFX TFX1000N is designed to deliver consistent, clinically optimized red light exposure to affected nails. Its single-spectrum architecture ensures precise energy delivery - uniform, predictable, and finely controlled.
Developed through photomedicine research, the TFX1000N provides reliable light distribution that supports localized tissue health and natural nail renewal.
Ideal for clinics seeking a dependable, high-performance phototherapy platform.
The ToeFX LT2000 integrates dual-band visible light technology designed to deliver complementary biological effects within the nail and periungual tissues. The system’s precisely calibrated spectral output ensures uniform light distribution and reproducible fluence across the treatment field. This harmonized approach enables broad-spectrum microbial disruption and supports endogenous tissue renewal processes - without heat, discomfort, or pharmacological agents.